| Field of application: | Enviromnmental technology/ Nisco Encapsulation Unit VAR J1 |
| User address: | EPFL- Ecole Polytechnique Fédérale de Lausanne LASEP-Soil & Environmental Physics Laboratory Secretariat GR A1 444 (Bâtiment GR) Station no. 2 CH - 1015 Lausanne (Switzerland) Tél. : +41 21 693 3775 |
We have been using alginate formulations to study the release of various toxic compounds, such as polyaromatic hydrocarbons (PAHs) and their subsequent bioavailability. In recent years the slow release of organic compounds exhibited by naturally occurring organic matter has attracted much attention in the controlled release literature for development of retarded diffusion release formulations that optimise the gradual disbursement of chemicals in agricultural use. This work has been helpful in our retarded release alginate formulations. We have been formulating barium alginate beads in which PAHs are entrained along with kaolinite filler. Spherical or bead shaped devices are the most useful in simultaneously approximating the shape of naturally occurring particles/aggregates with which we wish to draw comparisons, and being a straightforward geometry for diffusion models. Alginate beads that we have formulated display PAH release kinetics that are in excellent agreement with those found in the literature for naturally occurring materials such as soils and sediments.
A conventional phenomenological treatment of PAH release from sediments and soils is to fit a release curve with a biphasic exponential fit in which the fraction of material desorbed rapidly is a parameter obtained from the fit, is taken to represent the amount of bioremediable compound, and is assumed to reflect the portion of contaminant weakly sorbed to some putative labile fraction of sediment organic matter. Our work with model alginate systems illustrates deficiencies in this approach. Perhaps one of our more provocative findings resulted when we began working with beads sizes smaller than those achievable with the standard method of dropping the alginate solution from a conventional needle and syringe . Figure 1 compares the time-course of abiological release and biodegradation of naphthalene from 3.16 mm diameter beads to that of 570 µm diameter beads made with the Nisco VAR J1 bead maker, as well as the resulting biphasic exponential fits to the abiological data with the nominal predicted fraction of ‘bioavailable’ naphthalene.
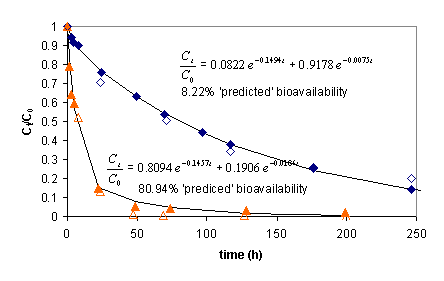
Figure 1. : Release of naphthalene from 3.16 mm diameter (blue diamonds) and 570 µm diameter (orange triangles) alginate beads. Solid symbols are from abiological desorption experiments using a quasi-infinite sink sorbent. Open symbols are from experiments wherein PAH degrading bacteria remove naphthalene. There are two interesting points about this diagram. The first is that the phenomenological model does not accurately represent true bioavailability, and the second is that the curve resulting from the smaller diameter alginate particles is virtually undistinguishable from similar curves for natural materials (unlike the curve resulting from larger diameter beads), implying that the characteristic biphasic release exhibited by natural materials may also result from particle size considerations rather than owing to some complex dynamical explanation involving n-fold different sorption compartments.
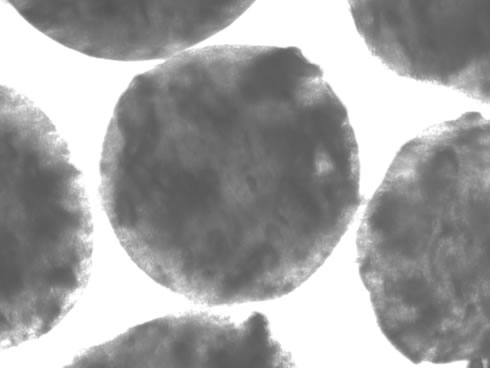
Figure 2. : Light microscope image of a typical 570 µm diameter alginate bead containing entrained naphthalene and kaolinite particles.
Figure 2 shows a light microscope image of a typical 570 µm diameter bead in which the alginate bead matrix is sufficiently transparent that the entrained particulates are visible. In other work we are entraining reporter bacteria into these smaller beads – the smaller beads being advantageous for situations wherein it is desirable to have target compounds and nutrients rapidly diffusing into the bead matrix.
