Cell immobilisation for the dairy industry
Introduction
Lactic acid bacteria (LAB) are widely used in the production
of fermented dairy products such as cheeses, yoghurts and
creams because of their technological, nutritional and eventual
health properties. The production of organic (mainly lactic
and acetic) acids and the resulting acidification is essential
for the production, development of typical flavour and preservation
of these products. The transformation of lactose by lactic
cultures improves the digestibility and various metabolic and
enzymatic activities of LAB lead to the production of volatile
substances, which contribute to flavour, aroma and texture
developments in fermented dairy products. Probiotics are defined
as microbial cells which transit the gastrointestinal tract
and which, in doing so, benefit the health of the consumer
(Stanton et al 2001). Among these
micro-organisms, LAB and especially lactobacilli and bifidobacteria
are already used in many probiotic dairy products.
Culture production and milk fermentations are traditionally
carried out in batch bioreactors using freely suspended microbial
cells. However, recent researches on immobilized cell (IC)
technology applied to LAB and probiotic cultures have emphasized
the importance and interest in this new technology. In particular,
cell immobilization has been shown to offer many advantages
for biomass and metabolite productions compared with free-cell
(FC) systems such as: high cell density and very high volumetric
productivity, reuse of biocatalysts, high process stability
(physical and biological) over long fermentation periods, retention
of plasmid-bearing cells, improved resistance to contamination,
uncoupling of biomass and metabolite productions, stimulation
of production and secretion of secondary metabolites and physical
and chemical protection of the cells.
Other complementary strategies to fermentation, based on cell
protection, have been tested to increase sensitive LAB, and
particularly bifidobacteria, survival in products and eventually
in the human intestine. These methods use cell encapsulation
to provide a physical barrier against the stressful environmental
conditions. This short review presents recent developments
in the immobilized cell technology with LAB and probiotic bacteria
and application in foods, with a focus in the dairy area. For
additional information, readers are invited to consult two
recent reviews on this topic (Doleyres and Lacroix 2004; Lacroix
et al 2004).
Immobilization techniques
Different methods (physical entrapment in polymeric networks,
attachment or adsorption to a preformed carrier, membrane
entrapment, microencapsulation) have been used for immobilizing
LAB. The purpose of these techniques is either to retain
high cell concentrations within the bioreactor or to protect
cells from a hostile environment. For industrial applications
in the food industry, the carrier material must be non-toxic,
readily available and affordable. It should also lead to
high-cell loading and the cells should have a prolonged viability
in the support.
For food applications, the most widely used immobilization
technique is the entrapment of cells within a food-grade
porous polymeric matrix (Table 1). In many applications,
controlled-size polymer droplets are produced using extrusion
or emulsification, under mild conditions. Thermal (κ-carrageenan, gellan,
agarose, gelatin) or ionotropic (alginate, chitosan) gelation
of the droplets are used to produce spherical gel biocatalysts.
These polymers are
readily available and widely accepted for use as additives
in the food and particularly dairy industry. Gel entrapment
is a relatively simple method resulting in usually spherical
beads with diameters ranging from 0.3 to 3.0 mm with high
biomass concentration. A careful selection of polymer composition
is necessary to achieve high mechanical stability of gel
biocatalysts during long-term fermentation, according to
the conditions of the fermentation (Artignan et al 1997).
However, the large scale production of beads under aseptic
conditions necessary for food applications still remains
an important issue for the industrialization of immobilized
cells in the food sector.
| Table 1: Lactic acid bacteria immobilization by entrapment techniques | |||
| Support | Species | Maximum cell concentration |
Reference |
| Ca-alginate | Lactococcus lactis ssp. |
2.0 10 |
Prévost and Diviès 1992 |
| Ca-alginate | Lactococcus lactis ssp. |
3.8 10 |
Prévost and Diviès 1992 |
| κ-carrageenan – LBG |
Lactobacillus casei | 5.1 10 |
Arnaud et al 1992 |
| κ-carrageenan – LBG |
Lactococcus lactis ssp. (three strains) |
1.3 10 |
Lamboley et al 1997 |
| Gellan gum | Bifidobacterium longum |
6.8 10 |
Doleyres et al 2002 |
Top or Bottom
Applications of immobilized cell technology
Biomass production
In this application, methods are used
to promote cell release from gel beads that occurs spontaneously
as microcolonies form near the surface of the biocatalysts.
In some applications, such as entrapped biomass production,
cream fermentations and metabolite productions, cell release
is not desirable. Steps for reducing FC levels have been presented
by Champagne et al (1994). However, repeated use of beads tends
to increase the level of FC, and although different treatments
have been tried, the rate of cell release is only reduced during
the early stages of the fermentation (Champagne et al 1994;
Klinkenberg et al 2001).
The release of cells growing in the peripheral layer of highly
colonized gel beads can be used to efficiently produce biomass
in the bulk liquid medium. This cell release activity can be
used for producing single or mixed strain cultures and to continuously
inoculate food liquids to process fermented foods such as fermented
milk products.
Lactic acid bacteria starter
The LAB are largely used in single and mixed cultures for the
production of cheeses, fermented milks, yoghurts, and cultured
butter. The main objectives of producing concentrated starters
are to obtain a high number of living cells containing the
necessary enzymes to function effectively during manufacturing
of cultured milk products, and also to maintain a given strain
balance in mixed starter cultures. Although lactic starters
are traditionally produced by batch fermentation, the accumulation
of metabolic end products
limits cell growth. The use of continuous fermentations may
overcome this limitation, but they are more susceptible to
contamination and loss of plasmid-mediated characteristics
and they rapidly lead to the disappearance or domination of
strains in a mixed-strain continuous fermentation (D’Angio
et al 1994; Gilliland 1985). One promising alternative is the
use of IC technology to produce mixed lactic starters in continuous
fermentation (Lamboley et al 1997). The high IC concentration
results in a very high productivity and decreases contamination
risks due to the high dilution and inoculation rates provided
by cell release from beads.
Immobilisation also improves plasmid stability (D’Angio
et al 1994; Huang et al 1996). The production of mixed-strain
mesophilic lactic starters was studied during continuous fermentations
of SWP, with three strains of lactococci separately immobilised
in κ- carrageenan/LBG gel beads in a stirred tank reactor
(Lamboley et al 1997). The process showed a high biological
stability and cell productivity (maximum cell productivity
of 5.3 10![]() cfu
l
cfu
l![]() h
h![]() )
over the tested period exceeding 50 days. By varying pH, dilution
rate (D) and temperature (T), a large range of strain ratios
could be obtained, while starter activity remained constant.
The microbiological and mechanical stabilities of gel beads,
that are critical properties of an IC process for industrial
applications, were shown during prolonged fermentations (7weeks).
)
over the tested period exceeding 50 days. By varying pH, dilution
rate (D) and temperature (T), a large range of strain ratios
could be obtained, while starter activity remained constant.
The microbiological and mechanical stabilities of gel beads,
that are critical properties of an IC process for industrial
applications, were shown during prolonged fermentations (7weeks).
Probiotic cultures
Probiotics are now used to prepare a large variety of pharmaceuticals
and fermented milk foods, such as fresh cheeses, fermented
milks and health supplements. However, many probiotic cultures,
such as bifidobacteria, are fastidious and non-competitive
bacteria that are very sensitive to environmental parameters,
such as oxygen and acidity, and require complex
and expensive media for propagation, with the addition of growth-promoting
factors, due to their stringent growth requirements (Ibrahim
and Bezkorovainy 1994).
Our recent data on IC technology clearly showed that this approach
can be used to continuously and stably produce mixed-strain
starters containing fastidious and noncompetitive micro-organisms,
such as bifidobacteria, with a high volumetric productivity
and high biomass concentrations in the outflow of the continuous
fermentation, even at high dilution rates exceeding the specific
growth rate (Doleyres et al 2004a). The production of a mixed
lactic culture containing Lactococcus lactic ssp. lactis biovar
diacetylactis MD and
Bifidobacterium longum ATCC 15707 was studied during a 17-day
continuous IC culture at different temperatures between 32
and 37°C. The two-stage
fermentation system was composed of a first reactor (R1) containing
cells of the two strains separately immobilized in κ-carrageenan/LBG
gel beads and a second reactor (R2) operated with free cells
released from the first reactor. The system allowed to continuously
produce a concentrated mixed culture with a strain ratio whose
composition depended on temperature and fermentation time.
A stable mixed culture (with a 22:1 ratio of L. diacetylactis
and B. longum) was produced at 35°C in the effluent of
R2, whereas the mixed culture was rapidly disbalanced in favour
of B. longum at a higher temperature (37°C) or L. diacetylactis
at a lower temperature (32°C). Cell growth in gel beads
is limited by diffusional limitations of both substrates and
inhibitory products, in this case lactic and acetic acids.
This leads to the development of steep inhibitory product,
pH and biomass gradients in colonized beads which can induce
a non-specific stress adaptation of IC. Data from Doleyres
et al (2004b) showed that IC technology combined with long-term
continuous culture can be used to efficiently produce, in a
one step process, cells
with enhanced tolerance to different environmental stresses.
In addition, cells produced by continuous IC cultures, which
are in exponential or early stationary growth phase, exhibited
both a high viability and metabolic activity compared with
starving cells produced by conventional batch cultures.
The IC technology can be used to continuously and stably produce
mixed-strain starters, eventually containing fastidious micro-organisms,
such as bifidobacteria, with a high volumetric productivity
and high biomass concentrations in the outflow of the continuous
fermentation, even at high dilution rates exceeding the specific
growth rate.
Prefermentation of Milk
Starter culture preparation is of paramount importance in the
manufacture of fermented dairy products. Any failure in the
starter preparation will, in most cases, lead to detrimental
effects on quality, affecting the appearance, texture and
flavour of the end product. Traditionally, batchwise processing
has been used, but increasing demand for these products has
resulted in
a large increase in size of fermentation tanks.
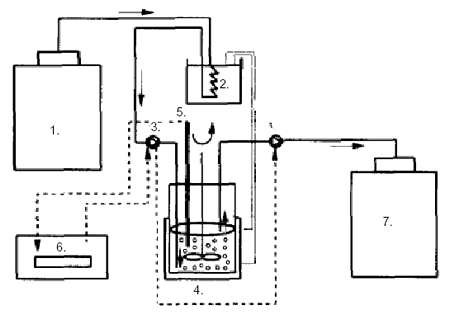
Figure 1:
Schematic
diagram of the apparatus for continuous inoculation and prefermentation
of milk:
1) refrigerated milk storage tank;
2) thermostated
water batch;
3) peristaltic pump;
4) continuously stirred tank reactor with lactic acid bacteria
immobilized in gel beads;
5) pH probe;
6) pH controller;
7) storage tank of preacidified
and inoculated milk
(from Sodini-Gallot et al 1995).
The continuous inoculation-prefermentation of milk for yoghurt
production in a stirred tank reactor by separately entrapped
cells of Lactobacillus delbruekii ssp. bulgaricus and Streptococcus
salivarius ssp. thermophilus in Ca-alginate gel beads was
the first studied dairy application of IC (Prévost and Diviès
1987; 1988a; 1988b). In this system, incoming milk was used
to control pH in the IC reactor (Figure 1). The milk feed rate
was therefore a direct function of the acidification activity
in the fermenter. With a pH set at 5.7, the residence time
of milk in the reactor was very short, 8-9 minutes, and lactic
acid production was increased 3.3-fold compared with free-cell
mixed cultures (Prévost
and Diviès 1988a). The ratio cocci:rod remained stable
in the prefermented milk during the 10 day culture, equal to
1 which is the optimum value for yoghurt manufacture. The high
cell confinement due to entrapment might explain the high cell
and lactic acid production in the bioreactor (Prévost
and Diviès 1988b). This technology allowed a reduction
in fermentation time by approximately 50 and 20% compared with
freeze-dried strains and a liquid yoghurt culture, and the
resulting yoghurt qualities were evaluated as satisfactory
(Prévost
and Diviès 1988b).
The inoculation-prefermentation of milk for fresh cheese
production with a mixed culture of entrapped in κ-carrageenan/LBG
gel beads was also extensively studied by our group. For
example, the total fermentation time to produce the fresh
cheese curd (pH 4.8) was considerably reduced by up to more
than 50% compared with the traditional industrial process
(Sodini et al 1995; 1997), or by 10-15% of that of batch
fermentation under optimum
laboratory conditions (Sodini et al 1998). This fermentation
time reduction was explained by the high inoculation level
and lower pH of prefermented milk compared with the classical
batch fermentation. Moreover, cells released from gel beads
are exponentially growing cells with no lag time compared with
freeze-dried or bulk starter cultures used in cheese manufacture.
The very high production rate (a reactor of 100 liters can
produce daily over
100,000 liters of prefermented milk inoculated with 1-2 10![]() cfu ml
cfu ml![]() ,
or 1 million liters of milk inoculated at the usual rate used
for fresh cheese production, i.e. approx. 10
,
or 1 million liters of milk inoculated at the usual rate used
for fresh cheese production, i.e. approx. 10![]() cfu ml
cfu ml![]() ), the
microbial stability and the extensive reduction of fermentation
times are among the major advantages of this new process.
), the
microbial stability and the extensive reduction of fermentation
times are among the major advantages of this new process.
Metabolite Production
Industrial cheese manufacturing yields
large volumes of whey as a by-product. In many plants, the
valuable whey proteins are concentrated by ultrafiltration
and incorporated into cheese or dried and sold as food ingredients.
The ultrafiltration step yields large volumes of low-value
whey permeate, which has limited uses. Due to its high lactose
(about 50 g l![]() ) and
) and
mineral contents (9 g l![]() ),
whey permeate may be used as a culture medium for the production
of lactic starter cultures or metabolites. The immobilized
cell technology has been studied by our group for production
of different metabolites and functional ingredients from LAB.
),
whey permeate may be used as a culture medium for the production
of lactic starter cultures or metabolites. The immobilized
cell technology has been studied by our group for production
of different metabolites and functional ingredients from LAB.
Lactic acid production
Lactic acid is widely used as an acidulant and preservation
agent in foods and as a precursor for production of emulsifiers,
such as stearoyl-2-lactylates, for the baking industries
(Vickroy 1985). New applications such as its use as a monomer
for production of biodegradable plastics and as an environment-friendly
chemical and solvent will increase future lactic acid
demand (Datta et al 1995). Whey permeate must be supplemented
with a source of metabolizable nitrogen and vitamins, such
as yeast extract (YE), in order to satisfy nutritional requirements
of LAB. However, the cost of YE contributes largely to lactic
acid production costs (Tejayadi and Cheryan 1995) and, therefore,
minimizing YE addition is an important goal for process optimization.
High lactic acid productivities and long-term stability (over
100 day periods) have been obtained during continuous IC
fermentation of YE-supplemented whey permeate by Lb. helveticus
immobilized in κ-carrageenan/LBG gel beads,
but with limited conversion of lactose (Norton et al 1994a).
Complete sugar conversion was only obtained when an additional
FC reactor was placed in series with the IC reactor (Norton
et al 1994b; Figure 2).
In this two-stage IC/FC process, an overall lactic acid productivity
of
13.5 g l![]() h
h![]() was
reached during continuous culture in whey permeate containing
10 g l
was
reached during continuous culture in whey permeate containing
10 g l![]() YE,
with 1 g l
YE,
with 1 g l![]() residual
sugar at an overall dilution rate of 0.27 h
residual
sugar at an overall dilution rate of 0.27 h![]() .
The authors also concluded that operation at low yeast extract
levels (1-3 g l
.
The authors also concluded that operation at low yeast extract
levels (1-3 g l![]() ),
or using periodic addition of higher YE concentrations,
),
or using periodic addition of higher YE concentrations,
could lead to a cost-effective operation. The productivity
of a two-stage IC reactor (19-22 g l![]() h
h![]() with
low residual sugar) largely exceeded that measured for the
two-stage IC/FC process (Schepers et al 2004) and for FC
batch cultures (approximately 2.0 g l
with
low residual sugar) largely exceeded that measured for the
two-stage IC/FC process (Schepers et al 2004) and for FC
batch cultures (approximately 2.0 g l![]() h
h![]() )
with the same strain and experimental conditions (Schepers
et al 2002). Higher productivities in SWP, with 10 g l
)
with the same strain and experimental conditions (Schepers
et al 2002). Higher productivities in SWP, with 10 g l![]() YE,
and high sugar utilization close to 100%, were only measured
in cell-recycle processes, with 35 g l
YE,
and high sugar utilization close to 100%, were only measured
in cell-recycle processes, with 35 g l![]() h
h![]() for
Lb. helveticus [9] and and 85 g l
for
Lb. helveticus [9] and and 85 g l![]() h
h![]() for
Lb. bulgaricus (Mehaia and Cheryan 1986). However, the industrial
application of membrane reactors is largely limited by high
capital investment, complex maintenance of the sophisticated
equipment and membrane fouling, particularly with whey permeate,
which results in a rapid
for
Lb. bulgaricus (Mehaia and Cheryan 1986). However, the industrial
application of membrane reactors is largely limited by high
capital investment, complex maintenance of the sophisticated
equipment and membrane fouling, particularly with whey permeate,
which results in a rapid
decrease of process performance (Tejayadi and Cheryan 1995).
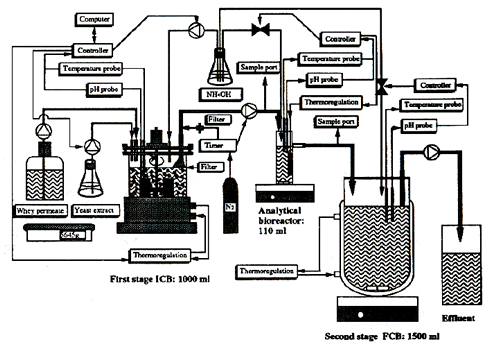
Figure 2:
Experimental set-up of the continuous two-stage fermentation process, including an intermediate analytical bioreactor. ICB = immobilized cell bioreactor; FCB = free cell bioreactor (from Norton et al 1994b).
Exopolysaccharide production
Exopolysaccharide (EPS) produced by LAB have received increasing
attention in recent years. They play an important role in
the manufacturing of fermented dairy products such as yogurts,
drinking yogurts, cheeses, fermented creams and milk-based
desserts. The EPS are very attractive for use as food additives
because of their contribution to the texture, mouthfeel,
taste perception and stability of the final product. They
may act as texturizers and stabilizers and so decrease syneresis
and improve product stability. Furthermore, EPS may contribute
to human health as prebiotics or due to their antitumor,
antiulcer, immunomodulating or cholesterol-lowering activities
(Ruas-Madiedo et al 2002). However, compared with dextran-producing
or Gram-negative EPS producers, the low production of EPS
by most LAB is a constraint for their commercial use as food
additives (De Vuyst and Degeest 1999).
Very little research has been done on cell immobilization for
EPS production. In our group, Bergmaier et al (2003) used the
mucoid properties of Lb. rhamnosus RW 9595M for cell immobilization
by adsorption on solid porous supports (ImmobaSil®).
The production of EPS was investigated during pH-controlled
IC repeated-batch cultures in SWP. A high immobilized biomass
of 8.5 1011 cfu ml-1![]() support
was measured after colonization by DNA analysis. During repeated
IC cultures, a high EPS concentration (1750 mg l
support
was measured after colonization by DNA analysis. During repeated
IC cultures, a high EPS concentration (1750 mg l![]() )
was measured after four cycles for a short incubation period
of 7 h. The high biomass in the IC system increased maximum
EPS volumetric productivity (250 mg l
)
was measured after four cycles for a short incubation period
of 7 h. The high biomass in the IC system increased maximum
EPS volumetric productivity (250 mg l![]() h
h![]() after
7 h culture) compared with FC batch cultures (110 mg l
after
7 h culture) compared with FC batch cultures (110 mg l![]() h
h![]() after
18 h culture corresponding to maximum EPS concentration of
1985 mg l
after
18 h culture corresponding to maximum EPS concentration of
1985 mg l![]() ).
A 38-day continuous fermentation was carried out in a two-stage
bioreactor under the same conditions, with a first fermentation
stage containing immobilized cells and a second fermentation
stage in series that was continuously inoculated by cells released
from the first reactor (Bergmaier et al 2004). Despite very
high biomass concentrations in both reactors, the average total
soluble EPS production of the fermentation system was very
low (138 mg l
).
A 38-day continuous fermentation was carried out in a two-stage
bioreactor under the same conditions, with a first fermentation
stage containing immobilized cells and a second fermentation
stage in series that was continuously inoculated by cells released
from the first reactor (Bergmaier et al 2004). Despite very
high biomass concentrations in both reactors, the average total
soluble EPS production of the fermentation system was very
low (138 mg l![]() ),
with no effect of temperature, dilution rate and carbon to
nitrogen ratio, compared with that measured in batch cultures
with FC (2350 mg l
),
with no effect of temperature, dilution rate and carbon to
nitrogen ratio, compared with that measured in batch cultures
with FC (2350 mg l![]() after
18 h incubation) or IC (1750 mg l
after
18 h incubation) or IC (1750 mg l![]() after
7 h) and continuous culture with FC (1808 mg l
after
7 h) and continuous culture with FC (1808 mg l![]() at
a dilution rate of 0.3 h
at
a dilution rate of 0.3 h![]() ).
This result was explained by important changes in cell morphology
and physiology, and the formation of very large aggregates
containing very high cell and insoluble EPS concentrations.
).
This result was explained by important changes in cell morphology
and physiology, and the formation of very large aggregates
containing very high cell and insoluble EPS concentrations.
These data show the high potential of the strain, Lb. rhamnosus
RW9595M, and of IC technology for the production of EPS as
a functional food ingredient. In addition, the production of
insoluble EPS allows an easy recovery of the product and the
aggregates containing high EPS and viable cell concentrations
could have interesting applications as symbiotic product, combining
both probiotic and prebiotic activities. Bacteriocin production
Bacteriocins are ribosomally-synthesized, extracellularly released
proteins or protein complexes with a bactericidal activity
against closely related bacteria, and for some bacteriocins,
against a wide range of Gram-positive bacteria (Jack et al
1995). In recent years, there has been considerable interest
in bacteriocins produced by LAB, and for diverse applications
such as biopreservatives, to improve quality and innocuity
of food products.
However, the major limiting factor in using bacteriocins as
food preservatives is their low yield during production. Cell
immobilization has been used to increase cell density for bacteriocin
production in supplemented whey permeate medium. A very high
nisin Z production (8200 IU ml![]() , with 1
, with 1
IU = 1 international unit of nisin = 0.025 µg pure nisin)
was measured in the broth after 1-h cycles during repeated-cycle
pH-controlled batch (RCB) cultures with Lactococcus lactis
ssp. lactis biovar. diacetylactis UL719 immobilized in κ-carrageenan/LBG
gel beads in SWP, with a corresponding volumetric productivity
of 5730 IU ml![]() h
h![]() or approximately 150 mg l
or approximately 150 mg l![]() h
h![]()
(Bertrand et al 2001). This productivity is much higher than
maximum nisin productivities reported in literature (approximately
500 IU ml![]() h
h![]() )
or maximum productivities obtained with the same strain UL719
for FC batch cultures (850 IU ml
)
or maximum productivities obtained with the same strain UL719
for FC batch cultures (850 IU ml![]() h
h![]() ),
and FC (460 IU ml
),
and FC (460 IU ml![]() h
h![]() )
or IC (1760 IU ml
)
or IC (1760 IU ml![]() h
h![]() )
continuous cultures. The stability of RCB cultures was demonstrated
)
continuous cultures. The stability of RCB cultures was demonstrated
for twenty-four and thirty-six 1-h cycles carried out over
3 and 6-day periods, respectively. A similar IC-RCB fermentation
was successfully used to produce a high concentration of pediocin
by P. acidilactici UL5 (Naghmouchi, 2003).
Microencapsulation
Probiotic bacteria are “living microorganisms,
which upon ingestion in certain numbers, exert health benefits
beyond inherent basic nutrition” (Guarner
and Schaafsma 1998). To produce therapeutic benefits, the minimum
suggested level of viable probiotic bacteria in a food product
should be ≥ 107 cfu ml-1![]() or
g
or
g![]() of a
product at the time of consumption (Adhikari et al 2000). Despite
the importance of viability of these beneficial organisms,
several surveys have shown large fluctuations and poor viability
of probiotic bacteria, especially bifidobacteria, in yogurt
preparations which is the main probiotic carrier (Schillinger
1999). Acidity, pH, concentration of lactic and acetic acids,
hydrogen peroxide, and dissolved oxygen content have been identified
to have an effect on viability during manufacture and storage
of yoghurt
of a
product at the time of consumption (Adhikari et al 2000). Despite
the importance of viability of these beneficial organisms,
several surveys have shown large fluctuations and poor viability
of probiotic bacteria, especially bifidobacteria, in yogurt
preparations which is the main probiotic carrier (Schillinger
1999). Acidity, pH, concentration of lactic and acetic acids,
hydrogen peroxide, and dissolved oxygen content have been identified
to have an effect on viability during manufacture and storage
of yoghurt
(Dave and Shah 1997). Moreover, because viable and biologically
active microorganisms are usually required at the target site
in the host, it is essential that the probiotic be able to
withstand the host’s natural barriers against ingested
bacteria. A microcapsule consists of a semipermeable, spherical,
thin, and strong membrane surrounding a liquid core, with a
diameter varying from a few microns to 1 mm (Gonçalves
et al 1992). Different materials have been used for microcapsulation
of LAB and probiotic
cultures (Table 2), although many of these technologies do
not fall within the strict definition of microencapsulation,
but rely on gel entrapment techniques. One important challenge
for cell encapsulation is the large size of microbial cells
(typically 1-4 µm) or particles of freezedried culture
(more than 100 µm). This characteristic limits
cell loading for small capsules or, when large size capsules
are produced, can negatively affect the textural and sensorial
properties of food products in which they are added. In almost
all cases, gel entrapment using natural biopolymers such as
calcium alginate, κ-carrageenan
and gellan gum has been favoured by researchers. However, although
promising on a laboratory scale, the developed technologies
for producing gel beads still present serious difficulties
for large-scale
production of food-grade microencapsulated microorganisms,
such as low production capacity and large bead diameters for
the droplet extrusion methods or transfer from organic solvents
and large size dispersion for the emulsion techniques, and
difficulty to maintain aseptic conditions.
Encapsulation of probiotic cells has been suggested by several
authors to enhance cell resistance to freezing and freeze-drying.
A higher stability was reported for Lb. acidophilus and B.
longum immobilized in alginate beads compared with FC during
storage of frozen dairy desserts (Shah and Ravula 2000) and
for B. longum in ice cream (Sheu et al 1993). In this latter
study, the addition of cryoprotective agents (glycerol and
mannitol) in the alginate
solution increased the protective effects of immobilization
and gave survival rates as high as 90% compared with only 40%
for FC. In addition, an optimum capsule size between 30 and
100 µm
was suggested for food applications, since a smaller diameter
resulted in less cell protection and a larger diameter gave
texture and sensory defects in the products. However,
ambiguous data were reported for immobilization and freeze-drying,
with a positive effect on cell viability for L. lactis (Champagne
et al 1992) and Lb. plantarum (Kearney et al 1990) immobilized
in Ca-alginate, but no effect on B. longum in κ-carrageenan/LBG
(Maitrot et al 1997).
Cell encapsulation also improved probiotic viability in fermented
dairy products. Immobilization of B. longum in κ-carrageenan
(Adhikari et al 2000), B. bifidum and B. infantis in Ca-alginate
(Hussein and Kebary 1999), and B. infantis in gellan-xanthan
beads (Sun and Griffiths 2000) allowed to maintain high-cell
concentrations during 5-week storage of yoghurt, with no change
in sensorial properties (Adhikari et al 2000). Picot and Lacroix
(2002) studied the encapsulation of B. breve and B. longum
as freeze-dried or fresh cultures in water-insoluble food-grade
microcapsules produced by emulsion and/or spray-drying, using
milk fat and/or denatured whey proteins as immobilisation material.
Viable counts of B. breve entrapped in whey protein microcapsules
using this method were significantly higher than those of FC
after 28 days in yoghurt stored at 4°C (+ 2.6
log cycles), but no effect was
observed for B. longum. The encapsulation of B. bifidum in κ-carrageenan
beads maintained cell viability for as long as 24-weeks of
cheddar cheese ripening, with no negative effects on texture,
appearance and flavor (Dinakar and Mistry 1994).
Top or Bottom
| Table 2: Microcapsules with potential use in the dairy industry. | ||
| Matrix | Species | Reference |
| Alginate | Lactobacillus plantarum | Kearney et al 1990 |
| Alginate | Lactobacillus delbruekii ssp. bulgaricus | Sheu et al 1993 |
| Alginate | Bifidobacterium bifidum Bifidobacterium infantis |
Hussein and Kebary 1999 |
| Alginate | Bifidobacterium longum | Lee and Heo 2000 |
| Alginate |
Lactobacillus acidophilus Bifidobacterium longum |
Shah and Ravula 2000 |
| Alginate | Lactobacillus acidophilus Bifidobacterium lactis |
Fàvaro Trindade and Grosso 2000 |
| κ-carrageenan | Bifidobacterium bifidum | Dinakar and Mistry 1994 |
| κ-carrageenan | Bifidobacterium longum | Adhikari et al 2000 |
| κ-carrageenan LBG | Bifidobacterium longum | Maitrot et al 1997 |
| Gellan/xanthan | Bifidobacterium infantis | Sun and Griffiths 2000 |
| Phtalate cellulose acetate |
Bifidobacterium pseudolongum | Rao et al 1989 |
Different encapsulation methods exhibited
protective effects on cell viability during in vitro tests
simulating gastric or intestinal digestion. Exposure to a simulated
gastric juice at pH 2.5 caused the FC viable count to drop
from 1.2 109 cfu ml-1 to undetectable levels in 30 min, while
the IC viable count decreased by only 0.67 log cycle within
the same period (Sun and Griffiths 2000). Lee and Heo (2000)
reported that survival of B. longum ATCC 15707
immobilized in calcium alginate beads in simulated gastric
juices and bile salt solution was better with higher gel concentrations,
with an effect of bead size. Very large beads (i.e. > 2
mm) provided more protection for B. longum ATCC 15707 in simulated
gastric juices and bile salt solution. Spray drying encapsulation
in whey-protein suspension protected B. breve, but not B. longum,
during and after sequential exposure to simulated gastric and
intestinal juices (+ 2.7 log cycles) compared with FC (Picot
and Lacroix 2002).
Different data obtained for the protective effects of immobilisation
might be due to strainspecific effects of immobilisation, but
also to differences in methodology of the different studies
since numerous experimental factors may affect cell survival.
In particular, the technological properties of cells should
be taken into account in the selection of encapsulation method,
such as heat resistance for spray-dray encapsulation.
Conclusions
Many advantages have been demonstrated for IC systems that
may be applied to LAB and probiotic bacteria in the dairy and
starter industries. However, no industrial application has
emerged yet. This can be explained by important changes in
the equipment and management of production, due to the switch
from batch to continuous fermentations. In addition, highly
qualified employees are certainly required to operate more
complex processes with IC and continuous or repeated batch
cultures that can run over 24 h daily periods. Application
of this research could be particularly important for the production
of probiotic bacteria, functional dairy products containing
high concentrations of viable bacteria and bioingredients from
LAB
with important functional properties for use in foods and health.
Immobilization can efficiently protect cells, making this approach
potentially useful for delivery of viable bacteria to the gastrointestinal
tract of humans via dairy fermented products. It may be anticipated
that application of IC technology in the dairy sector will
begin with these special cultures which
are difficult to propagate and use with the traditional culture
techniques, and which are used to produce high-value dairy
products with positive effects on consumers’ health.
